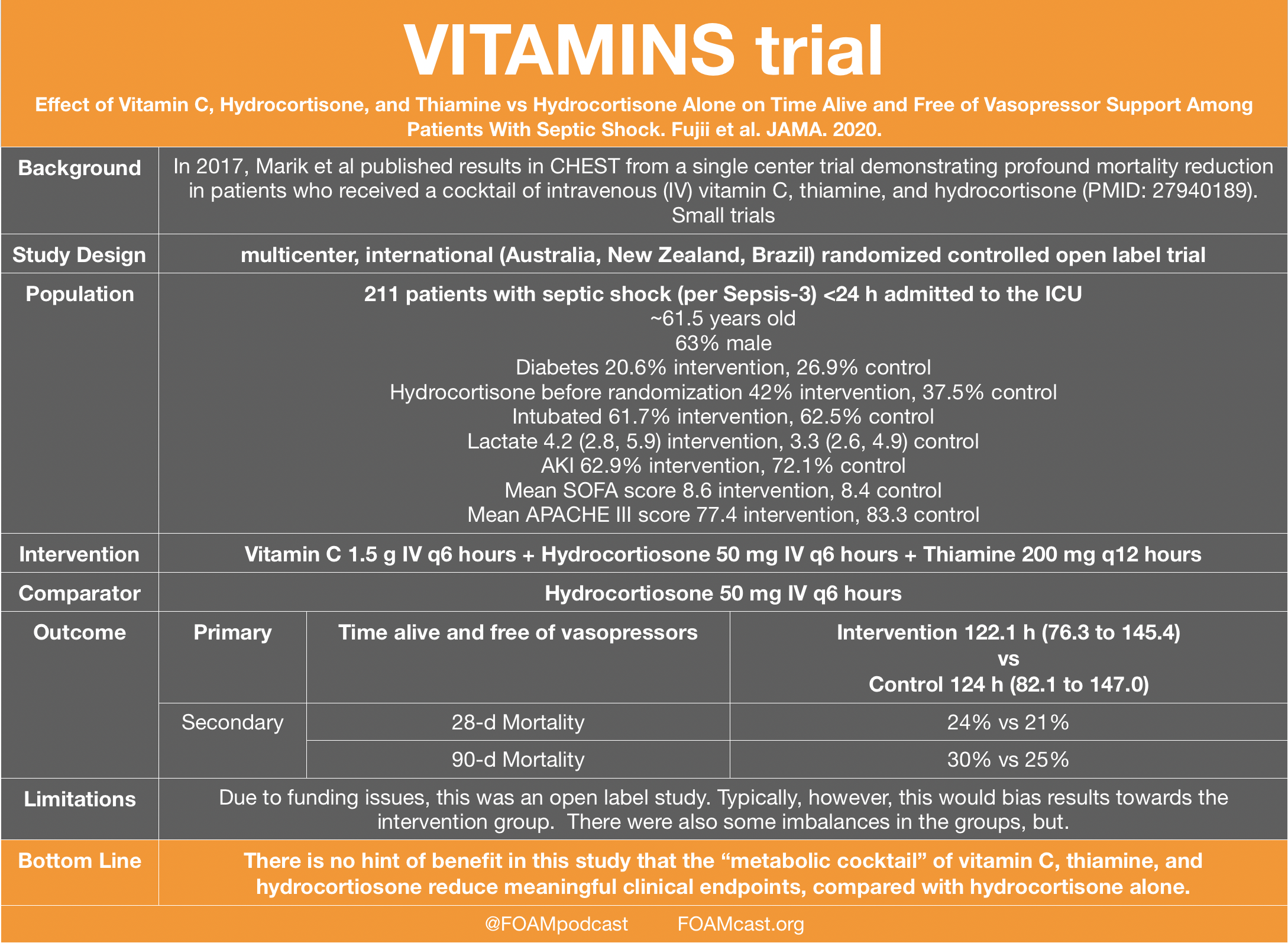
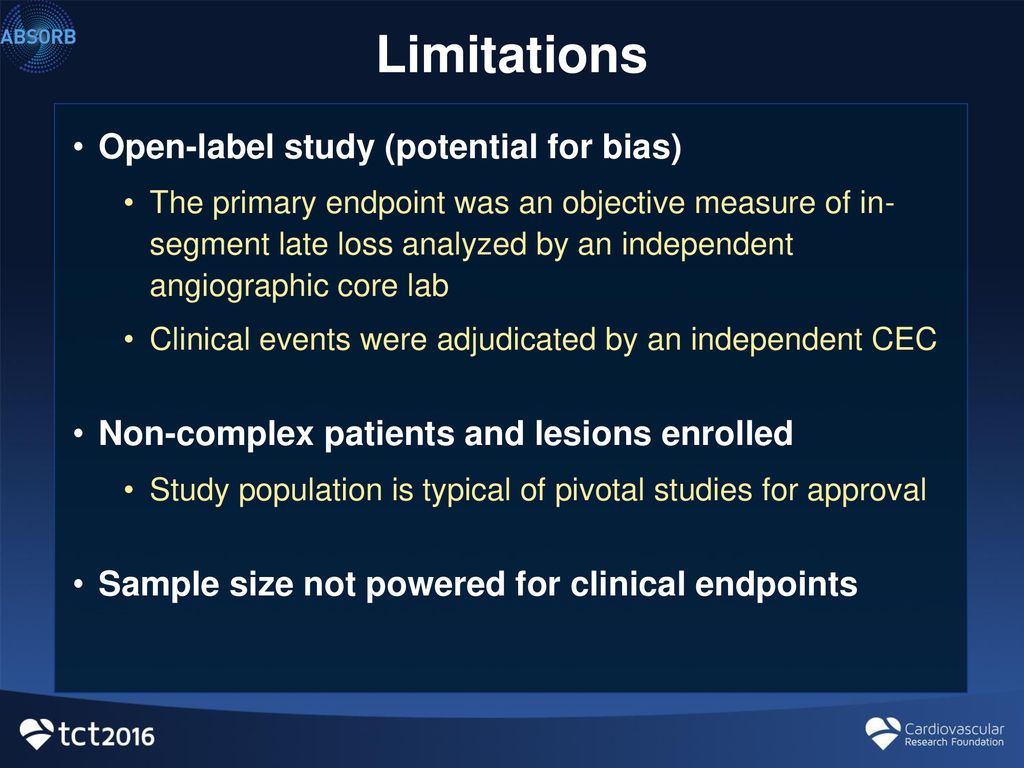
38 open-label study bias
Investigating the impact of open label design on patient‐reported ... Bias may occur in open-label trials, as observer bias and disappointment bias. 34 - 37 Therefore, according to the FDA, patients may be prone to provide biased reports of their own symptoms if they are aware of the treatment they received and lead to an overestimation of the treatment difference observed between the two treatment arms. Investigating Potential Bias in Patient-Reported Outcomes in Open-label ... While open-label bias is an important consideration for PRO results, bias related to knowledge of treatment assignment is not unique to PRO and may also affect other common trial outcomes, including progression-free survival 6 and clinician-reported safety data. For instance, the same forces that may lead patients to report fewer or milder ...
Case series - Wikipedia A case series (also known as a clinical series) is a type of medical research study that tracks subjects with a known exposure, such as patients who have received a similar treatment, or examines their medical records for exposure and outcome. Case series may be consecutive or non-consecutive, depending on whether all cases presenting to the reporting authors over a …

Open-label study bias
Elsevier Health Hier sollte eine Beschreibung angezeigt werden, diese Seite lässt dies jedoch nicht zu. Open-Label Extension Studies | SpringerLink Open-label extension studies have been described as "case series [studies] of the survivors of the [double-blind] trials", [ 1, 2] inferring a range of deficiencies and potential biases in their design and interpretation, issues which will be discussed in this article. Understanding Clinical Trial Terminology: What is an Open Label ... Alternatively, sometimes, trials are conducted in an open-label fashion, meaning study participants and researchers both know which treatment the patient is receiving. Open-label trials can be used to compare treatments or gather additional information about the long-term effects in the intended patient population.
Open-label study bias. Home Page: Biological Psychiatry Special Issue Call for Papers: Metabolic Psychiatry. There is robust evidence about the critical interrelationships among nutrition, metabolic function (e.g., brain metabolism, insulin sensitivity, diabetic processes, body weight, among other factors), inflammation and mental health, a growing area of research now referred to as “Metabolic Psychiatry.” (PDF) What is an open label trial? - ResearchGate An open label randomised controlled trial study design was used. The control treatment was prazosin alone. The setting was a hospital and research centre in Mahad, a region of India. Participants... Exploring open-label bias in patient-reported outcome (PRO) emotional ... e18702 Background: Concern exists that patients' self-reports may be biased in open-label trial designs. We compared PRO emotional domain results between investigational arms of paired open label and double-blind trials of the same drug and disease population. We hypothesized that greater improvement in emotional domain scores would be found in the investigational arms of open label compared ... External and internal validity of open label or double‐blind trials in ... Naturally, in open-label trials in anticoagulation there is a risk of a reporting bias of adverse events. Patients may research the new drug and its side-effects in publications and may be influenced in their reporting behaviour of potential side-effects. Furthermore, investigators may be equally susceptible to a reporting bias.
Open-label trial - Wikipedia An open-label trial, or open trial, is a type of clinical trial in which information is not withheld from trial participants. [1] In particular, both the researchers and participants know which treatment is being administered. [1] National Cancer Institute NCI's Dictionary of Cancer Terms provides easy-to-understand definitions for words and phrases related to cancer and medicine. Login to your account - The Lancet Respiratory Medicine Jun 27, 2022 · We did an open-label, prospective, two-arm, parallel-group, multicentre, randomised, controlled, superiority trial in 26 hospitals in the UK. We recruited adults from rheumatology and dermatology clinics who had been diagnosed with an immune-mediated inflammatory disease (eg, rheumatoid arthritis, psoriasis with or without arthritis, axial spondyloarthritis, atopic dermatitis, polymyalgia ... Reducing bias in open-label trials where blinded outcome assessment is ... Many trial designs do not permit blinding, and are therefore designed as open-label, with patients, clinicians, and other study investigators aware of treatment allocation. Research has suggested that these trials should use blinded outcome assessment to avoid bias in estimated treatment effects [ 6 - 10 ].
Some Blinding Techniques in Clinical Trials - Blogger The firewall between the sponsor the investigator/clinical research organization can also be implemented in an open label study or single-blind study so that the sponsor is prevented to access the cumulative data for the primary efficacy endpoint for analysis. ... Brennan C Kahan (2014) Reducing bias in open-label trials where blinded outcome ... Bias was reduced in an open-label trial through the removal of ... Objective: To determine whether modifying an outcome definition to remove subjective elements reduced bias in a trial that could not use blinded outcome assessment. Study design and setting: Reanalysis of an open-label trial comparing a restrictive vs. liberal transfusion strategy for gastrointestinal bleeding. The usual definition of the primary outcome, further bleeding, allows subjective ... What is an Open-Label Clinical Trial? - News-Medical.net Open-label trials are insufficient for providing data on these reactions. Open-label trials can increase the confidence about incidence rates, but as they are typically biased and uncontrolled,... Statistical controversies in clinical research: limitations of open ... However, the bias apparently concerns open-label as well as DB trials, suggesting that asymmetry was not related to the design of the missing trials. Analysis by study design showed an OR for bleeding of 3.18 (95% CI 2.65-3.81) for open-label studies and 1.89 (95% CI 1.63-2.19) for DB studies . The ratio of these two ORs summarizes the ...
Open label extension studies and patient selection biases An alternative method of analysis is proposed, which does not rely on the often unjustifiable assumption of outcomes being missing completely at random. Results: In an example open label extension study, with reported responder rate 43%, we show how an analysis allowing for patient selection biases produces a responder rate of just 28%.
Non interventional study - vjcgw.drillraps.shop 06.09.2022 · Describe any measures taken to eliminate bias. State the study duration/timeline. If there is incomplete disclosure, deception, placebo, or a sham procedure, provide the rationale, the process, and any de-briefing measures. . A prospective, non-interventional, multicentric cohort study Leif Erik Sander, MD Chair of Infectious Diseases Department of Infectious Diseases and …
Co-creation using crowdsourcing to promote PrEP adherence in … Sep 07, 2022 · Incorporating those measures may mitigate the impact of the bias. Our study may help to address important policy and research questions. The study outcomes may help to guide policy and intervention practices among community-based organizations and medical system regarding facilitating adherence to PrEP uptake among key populations.
Open label extension studies: research or marketing? - PMC Open label extension studies are a common adjunct to double blind randomised controlled trials of new drugs. The aim of open label extension studies often seems to be to enable continued use of a new drug for marketing or compassionate purposes rather than to increase knowledge. The continued use of a new drug on compassionate grounds should ...
Consent to open label extension studies: some ethical issues Patients are openly given the active substance at this stage, regardless of their assignment in the initial trial. Investigators are typically reluctant to unblind the patients' assignment at the point of entry into the open label phase, on the grounds that this may introduce ascertainment bias in the main study.
Clinical study design - Wikipedia Ecological study; Important considerations. When choosing a study design, many factors must be taken into account. Different types of studies are subject to different types of bias. For example, recall bias is likely to occur in cross-sectional or case-control studies where subjects are asked to recall exposure to risk factors. Subjects with ...
Open-Label Extension Studies | SpringerLink Open-label extension studies (OLEs) create a number of challenges for data analysis and interpretation. These challenges include bias in outcomes and adverse clinical events that may exist because of subject selection and participation.
Open label extension studies and patient selection biases Results In an example open label extension study, with reported responder rate 43%, we show how an analysis allowing for patient selection biases produces a responder rate of just 28%. Conclusions ...
What is an open label trial? | The BMJ Open label trials are sometimes referred to as "non-masked" or "unblinded." If the trial is a non-pharmacological study, such as a trial of devices, or psychological and physical treatments, it may be referred to simply as "open." After recruitment to the trial, the participants were allocated to treatment using block randomisation.
PDF Blinding Sponsors for Open Label Studies: Challenges and Solutions - MWSUG Even for open label studies, it's still desirable blind the study sponsor to reduce potential bias due to the sponsor knowing the treatment level aggregated data while the study is ongoing. The practice helps increase credibility of study results and thus should be followed, particularly for registration studies. In open label studies, in ...
PDF RESEARCH ETHICS Consent to open label extension studies: some ethical ... of society. The situation I have in mind is the open label extension study, a relatively common type of drug trial. A recent MEDLINE search for "open label extension studies", limited by publication type to randomised controlled trials, produced 55 references between 1992 and 2000. Open label extension studies typically follow on from (and ...
Adjuvant atezolizumab after adjuvant chemotherapy in ... - The Lancet 20.09.2021 · guidelines were adhered to in this study to ensure standard patient care and minimise the potential bias of the open-label design. The frequency and types of scans were consistent with those of a global trial, and the study protocol allowed for any patient to have additional scans as clinically indicated, done according to the protocol. A placebo arm was not …
Bias for Patient-Reported Outcomes in Open-Label Cancer Trials: How Big ... A common concern with patient-reported outcomes (PROs) in open-label trials is that a patient's knowledge of treatment received could influence their view and reporting of their symptoms. With this in mind, members of the US Food and Drug Administration explored the possibility of such bias in a recent viewpoint published in JAMA Oncology.
Effects of open-label placebos in clinical trials: a ... - Nature Open-label placebos (OLPs) are placebos without deception in the sense that patients know that they are receiving a placebo. The objective of our study is to systematically review and analyze the...
Open-Label Trial - an overview | ScienceDirect Topics Open-label studies lack the rigor of blinded studies. Since the lack of blinding can introduce significant bias, reserve the use of open-label studies for situations in which blinding is neither feasible nor ethical or in cases where the outcome is completely objective, such as survival. Some situations include: •
Detection bias in open-label trials of cancer drug: a meta ... Satoshi Funada added file 20210625_protocol_draft_1.pdf to OSF Storage in Detection bias in open-label trials of cancer drug: a meta-epidemiological study 2021-06-25 12:19 AM Satoshi Funada removed file 20210622_protocol_draft_1.pdf from OSF Storage in Detection bias in open-label trials of cancer drug: a meta-epidemiological study
Identifying and Avoiding Bias in Research - PMC - National Center for ... Well designed, prospective studies help to avoid. selection bias as outcome is unknown at time of enrollment. Channeling bias. • Assign patients to study cohorts using rigorous criteria. Bias during trial. Interviewer bias. • Standardize interviewer's interaction with patient. Blind. interviewer to exposure status.
PDF A Phase 3 Open-label, Multicenter, Randomized Study of ASP2215 versus ... A Phase 3 Open-label, Multicenter, Randomized Study of ASP2215 versus Salvage Chemotherapy in Patients with Relapsed or Refractory Acute Myeloid Leukemia (AML) ... or accumulation of substantial amount of data in an open-label study to ensure lack of bias. For operational efficiency an earlier time is usually targeted and wherever possible, the ...
Design characteristics, risk of bias, and reporting of randomised ... In trial CA204-009, which was open label, outcome assessors were aware of the intervention received by study participants, and assessment of the progression free survival outcome could have been influenced by knowledge of the intervention received. ... Furthermore, our risk of bias assessments were not blinded to study results because risk of ...
Understanding Clinical Trial Terminology: What is an Open Label ... Alternatively, sometimes, trials are conducted in an open-label fashion, meaning study participants and researchers both know which treatment the patient is receiving. Open-label trials can be used to compare treatments or gather additional information about the long-term effects in the intended patient population.
Open-Label Extension Studies | SpringerLink Open-label extension studies have been described as "case series [studies] of the survivors of the [double-blind] trials", [ 1, 2] inferring a range of deficiencies and potential biases in their design and interpretation, issues which will be discussed in this article.
Elsevier Health Hier sollte eine Beschreibung angezeigt werden, diese Seite lässt dies jedoch nicht zu.


![PDF] Safety of oral ivermectin during pregnancy: a systematic ...](https://d3i71xaburhd42.cloudfront.net/4e1f7ccf234e029667ee9c730262ec03978ba2bd/5-Table3-1.png)


















Post a Comment for "38 open-label study bias"