44 open-label study advantages and disadvantages
External and internal validity of open label or ... - Wiley Online Library Naturally, in open-label trials in anticoagulation there is a risk of a reporting bias of adverse events. Patients may research the new drug and its side-effects in publications and may be influenced in their reporting behaviour of potential side-effects. Furthermore, investigators may be equally susceptible to a reporting bias. External and internal validity of open label or double-blind trials in ... disadvantages of open-label or double-blind trials is currently underway and interpretation oftrialresults isoften focusedon this matter. In general, a blinded trial is regarded as being less subject to bias than an open trial because it minimizes the impact of knowledge of treatment allocation on post-random-
14 Advantages and Disadvantages of a Randomized Controlled Trial It makes groups become comparable through the collected data according to known and unknown factors that investigators find during the research process. Even a blocked randomization effort can make groups comparable when they fall within known confounding factors. 4. It offers a higher level of statistical probability.
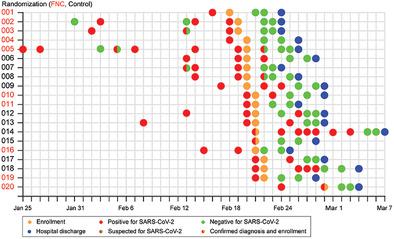
Open-label study advantages and disadvantages
Open-label study | definition of open-label study by Medical dictionary open-label study: a study in which there is no blinding of treatments. PDF What Are Open-Label Extension Studies For? In open-label assessment studies, there is a sig-nificant risk of biased assessment. Analysis of all subjects who were randomized (intent to treat analysis) is another important technique, since subjects who drop out can differ in crucial ways from subjects who remain in the study. In open-label extension studies, only a proportion of the sub- Advantages and disadvantages of randomised control study design The candidates would have scored highly by discussing the following issues: Treatment allocation Randomisation to eliminate selection bias Adequate sample size to achieve power Logistics of conducting multi-centre trials Blinding Prospective design Trials of efficacy versus effectiveness Applicability Ethical considerations
Open-label study advantages and disadvantages. Open-Label Trial - an overview | ScienceDirect Topics Open-label studies lack the rigor of blinded studies. Since the lack of blinding can introduce significant bias, reserve the use of open-label studies for situations in which blinding is neither feasible nor ethical or in cases where the outcome is completely objective, such as survival. Some situations include: • NCI Dictionary of Cancer Terms NCI's Dictionary of Cancer Terms provides easy-to-understand definitions for words and phrases related to cancer and medicine. External and internal validity of open label or double-blind trials in ... a blinded trial may be less subject to bias than an open-label trial because it minimizes the impact of knowledge of treatment allocation on postrandomized treatment decisions and on reporting of... Understanding Clinical Trial Terminology: What is an Open Label ... Open-label trials can be used to compare treatments or gather additional information about the long-term effects in the intended patient population. In some instances, patients who complete one clinical trial may be eligible to continue in an open-label extension study where all participants are eligible to receive active treatment for an ...
Open-label extension studies: do they provide meaningful information on ... Increased human exposure to a new medicine under reasonably controlled circumstances to increase confidence in the safety of the medicine is an acceptable rationale for an open-label extension study, and a useful activity to increase the knowledge of the safety profile of a new medicine. External and internal validity of open label or double-blind trials in ... In general, a blinded trial is regarded as being less subject to bias than an open trial because it minimizes the impact of knowledge of treatment allocation on post-randomized treatment decisions and on reporting of outcomes. However, a blinded trial is not always feasible. (PDF) What is an open label trial? - ResearchGate Researchers assessed the effectiveness of prazosin combined with scorpion antivenom in assisting recovery from scorpion sting. An open label randomised controlled trial study design was used. The ... What is an open label trial? | The BMJ Open label trials are sometimes referred to as "non-masked" or "unblinded.". If the trial is a non-pharmacological study, such as a trial of devices, or psychological and physical treatments, it may be referred to simply as "open.". After recruitment to the trial, the participants were allocated to treatment using block randomisation.
Advantages and Disadvantages with Labelling Machines The device then shrinks back and the label is attached to your product. The advantage of using these types of labelling machines is that precision is excellent. This method is great when labelling difficult packaging products. The disadvantage is that this method of labelling can be slow and the labelling quality not as good as other methods. Advantages and disadvantages of randomised control study design The candidates would have scored highly by discussing the following issues: Treatment allocation Randomisation to eliminate selection bias Adequate sample size to achieve power Logistics of conducting multi-centre trials Blinding Prospective design Trials of efficacy versus effectiveness Applicability Ethical considerations PDF What Are Open-Label Extension Studies For? In open-label assessment studies, there is a sig-nificant risk of biased assessment. Analysis of all subjects who were randomized (intent to treat analysis) is another important technique, since subjects who drop out can differ in crucial ways from subjects who remain in the study. In open-label extension studies, only a proportion of the sub- Open-label study | definition of open-label study by Medical dictionary open-label study: a study in which there is no blinding of treatments.





Post a Comment for "44 open-label study advantages and disadvantages"